- Nonclinical Contract Research TOP
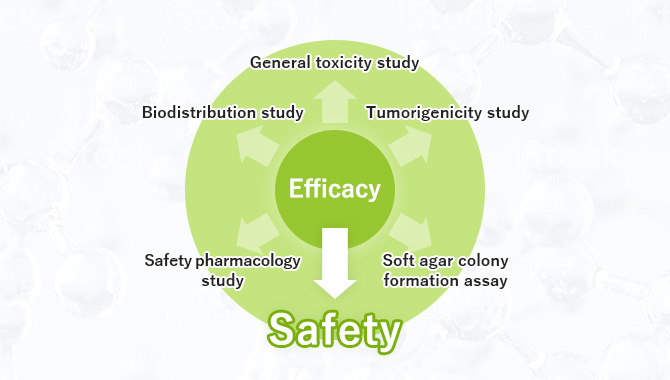
- Safety Studies
- Genotoxicity Studies
- Safety Pharmacology Studies
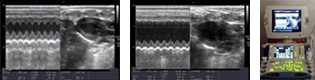
- Pharmacology Studies
- Pharmacokinetic Studies
- Characterization Studies of Test Substances, Concentration Analysis of Formulations
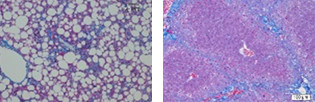
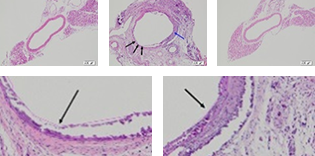
- Pathological Examination
- Consulting/Advisory Services
- Education and Training
- Medical Writing
- Topics TOP
- Patient-Derived Xenografts (PDX) for Drug Development
- Patient-Derived Cells (PDC) for Drug Development
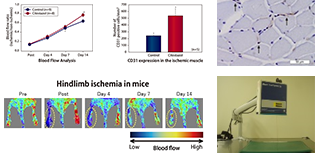
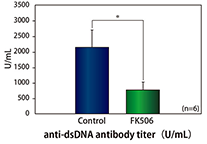
- Regenerative Medicine Products Development Support
- Mass Spectrometry Imaging
- Introduction of PBMC isolation and analysis service
- Approach to analyzing nucleic acid pharmaceuticals
- Introduction of analytical methods for novel modalities