Central Laboratory Services
Centralized testing with unified platform and reference ranges for efficient data analysis

We perform centralized testing for clinical trial samples from nationwide medical institutions. This avoids any potential platform discrepancies between institutions, unifying testing methodologies and reference ranges.
We are also able to address our clients’ drug development needs in terms of globalization through mediford’s global laboratories network.
Service Process
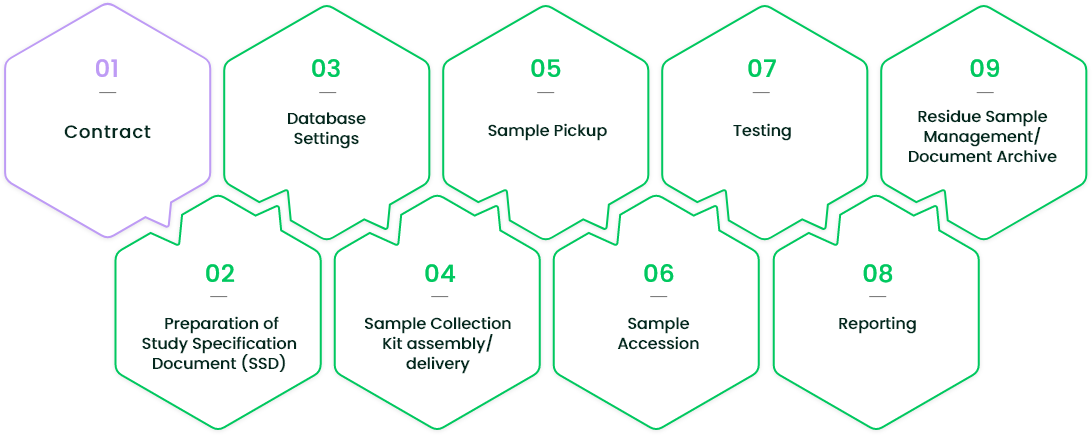
Our central laboratory services are available in four stages: Contract, Initiation, Operation, and Completion.
Stage 1Contract stage
For consultations regarding new clinical studies, mediford's sales representatives will be the first contact and manage everything from quote preparation to conclusion of contracts. When the contract is signed and study is commissioned, a Project Manager is appointed for each study and will manage the entire project until its completion.
The flow after the commission:
- Preparation of SSD (Study Specification Document)
- Settings up in the core system
- Assembly of sample collection materials
- Pickup of submitted sample
- Accession of sample
- Performing of testing
- Reporting of results
- Storage of residue sample after testing, study-related documents archives.
We will also ship sample to designated laboratories domestically and internationally upon request.
Stage 2Initiation stage
During the project initiation stage, we assign Study Design Personnel for each study. These individuals will design the entire project according to the client's requests, and will configure mediford's core system, e-MADAM (e-Mediford Corporation Advanced Data Management System) accordingly. They also create the “Study Specification Document” (SSD) based on the contents requested by the client.
In SSD, several key factors as follows are determined and described:
- The combination of blood collection tubes according to the tests scheduled for each visit (“visit” refer to the point at which samples are collected from participants/subjects in clinical studies)
- The contents and format of test requisition form and report, and
- Sample shipment details to designated laboratories in Japan or internationally.
Once the SSD is finalized after being confirmed by the client, the contents will be registered in e-MADAM. Contents include demographics of the clinical study participants (such as gender and age), test items, visits (points for sample collection), medical institutions conducting the study, and configuration of sample collection material sets.

Stage 3Operation stage
Once the system registrations are complete, projects will enter the operational stage.
Each project is performed by specialized Study Operations Personnel who have been previously assigned to the project.
In the operation stage, these individuals carry on daily operational tasks, such as reporting test results to the client and medical institutions conducting the study, responding to inquiries related to sample pickup and submission, internal and external coordination, and specimen shipment to international facilities.
Stage 4Completion stage
When the study period ends and we receive a study termination notification from the client, the project will enter the completion stage.
At the client’s request, mediford will archive test-related documents on a contract basis, as well as discard or otherwise process samples stored during the study period.




