Cell Characteristic Analysis

Characteristic antigens (proteins, sugar chains) are expressed on the surfaces of cells in biological samples and cultured cells. By detecting these surface antigens with specific antibodies, it becomes possible to analyze the features and roles of the cell groups.
For example, by analyzing lymphocyte groups in blood, you can estimate the immune status of the whole body, and population shifts of immune cells induced by drugs and functional foods. By analyzing cell groups obtained from tumor tissues, it is possible to analyze the diversity of the whole small area. Cytokine production ability can also be evaluated by stimulating and cultivating specific cell groups.
Mediford Corporation offers cell characteristic analysis service by flowcytometry, mass cytometry, and ELISpot, as well as cell isolation services of specific cell groups with cell sorters.
Service Details
Flow cytometry
Flow cytometry (FCM) is a technique for analyzing cell characteristics in non-uniform mixtures like blood. It is possible to analyze cell surface markers or intracellular antigens by utilizing fluorescence-labeled antibodies.
We have BD FACSCant II (8 colors) and BD FACSLyric (12 colors) equipped with 3 types of lasers from BD Biosciences.
Besides blood (whole blood), peripheral blood mononuclear cells (PBMC), and culture cells, you can select as many samples as needed. In addition to commercial kit products, panels internally validated and specified by the sponsor are available. We have extensive experience in evaluating drug stimulation response and analyzing receptor saturation, as well as contracting with pharmaceutical and beverage companies.


You can establish analytical methods which meet your needs in consultation with our trained scientists. Please feel free to consult us.
Mass cytometry (CyTOF analysis)
Mass cytometry is a combined technique of flow cytometry which analyzes cells in homogenate and ICP-TOF-MS which detects cells binding to metal stable isotope labeled antibodies.
We have introduced cytometry by time-of-flight (CyTOF XT) from Standard BioTools which can analyze more than 40 targets from a single sample. Compared with flow cytometry, CyTOF can obtain more stable results because there is no discoloration of pigments or leakage of lights. We can propose the best method while taking into consideration the characteristics of the techniques and the aim of analysis.

ELISpot
Enzyme-linked immunospot (ELISpot) is an immunoassay with very high sensitivity which can detect cytokines secreted from a single cell. As the cytokines secreted from cells combine with antibodies immediately, this technique is free from any influence of degradation or uptake into cells. Cytokines are trapped by antibodies on the well-bottom layer, then react with secondary antibodies or enzymes, and form cytokine spots at the position of the well-bottom layer where the cells are located. Cytokines producing cells are visualized and determined by counting the spots.
We have Immunospot Analyzer from CTL which is used for determination of interferons and interleukins with kits from CTL and Mabtech.
We have extensive experience in efficacy evaluation studies for development of vaccines for infection and cancer, and cell pharmaceuticals.


Cell based assay
Cell based assay is a technique for evaluating activity and toxicity of drugs and objective compounds utilizing cells. In pharmaceutical development, it is used for evaluating concentration and titer of dosing drugs in body and evaluating antidrug antibodies after dosing protein formulations. Bioassay is utilized to verify the effectiveness in the development of vaccines as well.
We have extensive experience in neutralization antibody titration to dosing drugs and evaluation of immune response and immunogenicity in vaccine development.
PBMC isolation
Peripheral blood mononuclear cells (PBMC) consist of various immune cells such as T cells, B cells, NK cells, monocytes, and dendritic cells, etc. Recently it has attracted attention as it is used for many purposes such as immunological research and development of vaccines.
In general, PBMCs are separated and collected from fresh blood by density-gradient centrifugation. Layering blood on density solution and collecting/washing separated PBMCs is performed manually, and it requires a high level of skill to obtain PBMCs with high quality and homogeneity. Adequate staff organization for minimizing the duration between blood collection and PBMC isolation is necessary to obtain PBMCs that closely reflect in-vivo conditions.
Mediford Corporation has a long history of experience in PBMC isolation in clinical trials. We pick up blood samples from study sites, and highly skilled staffs perform the PBMC isolation process at the GLP laboratory located in Itabashi, Tokyo. In addition to the standard process, we can utilize other simpler isolation technologies such as Vacutainer CPT from BD, Leucosep tube from Greiner, SepMate from STEMCELL Technologies.
With isolated PBMCs, we can conduct analysis by flow cytometry, ELISpot, and qPCR. In addition, we can conduct long-term storage for future studies, or shipment to other laboratories.
A case study of PBMC isolation with ficoll is shown below.
A case study of PBMC isolation with ficoll


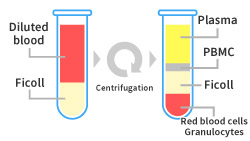




Please contact us if you are interested in watching a video explaining the PBMC isolation process from blood samples.
Pathogen (bacteria, fungus, virus etc.) related analysis
Detection and analysis of related pathogens are often conducted in epidemiological surveys and development of antimicrobial drugs, antiviral drugs, and vaccines.
Our track record with pathogens under reliability standard regulations is listed below
- Separation/Identification or titration by bacterial/viral culture
- Drug sensitivity determination with separated/identified strain
- Identification and quantification of nucleic acids by PCR, etc.
- Genome analysis by sequencing (including NGS) (Classification of pathogens in details in epidemiological surveys, Confirmation of drug tolerance by identifying the position of mutation)
- Antibody titration by viral culture or hemagglutination
The list above is an example, and the suitable method differs depending on the pathogen analyzed. Please feel free to consult us about pathogens not listed above.