Globalization in Drug Development

Mediford Corporation utilizes its overseas laboratories network to respond to the globalization of clinical studies.
Quality management of outsourced services is secured by our specialized overseas laboratory management department.
Service Details
Support for globalization in drug development
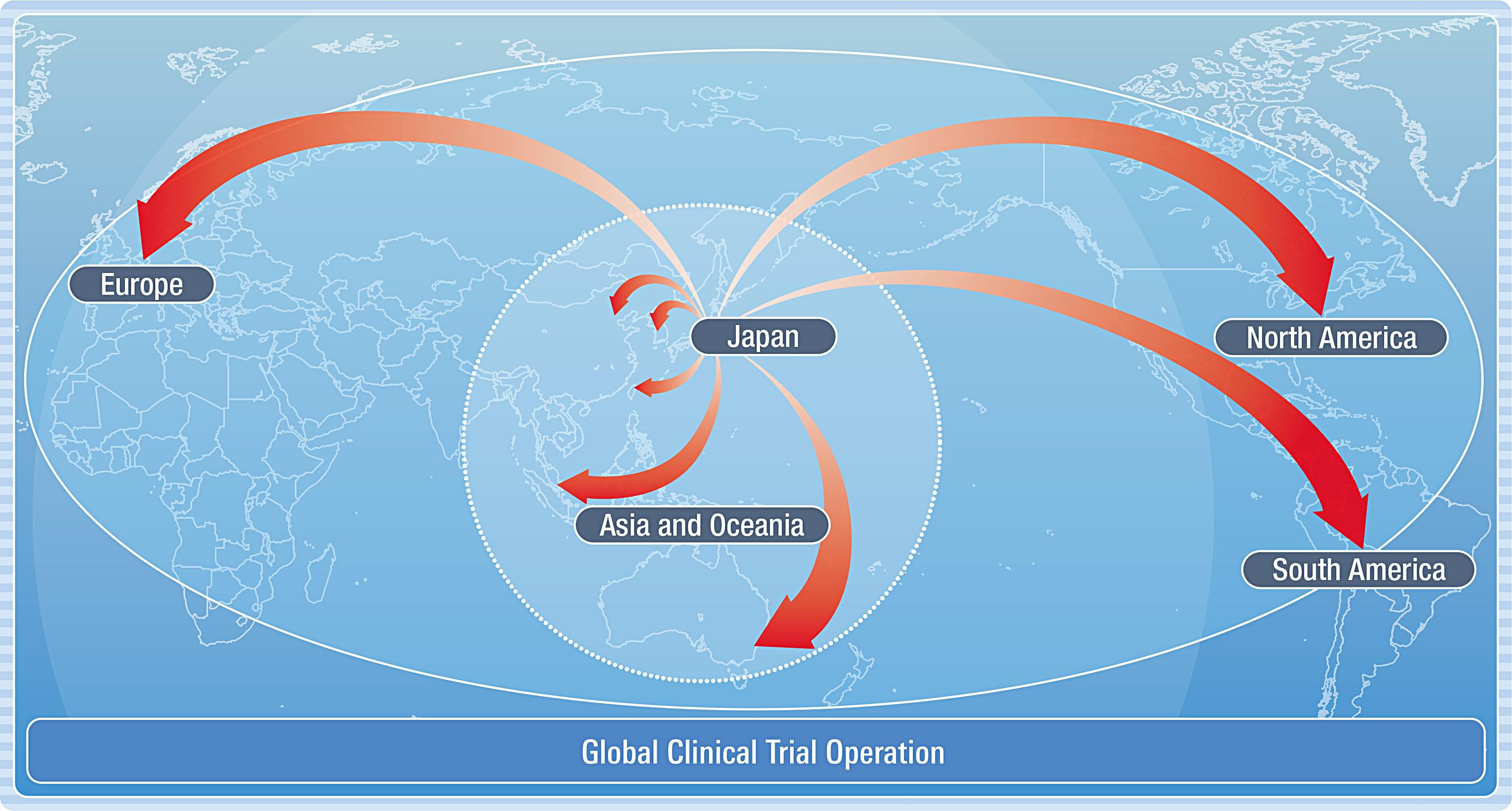
Mediford Corporation supports global clinical trials.
For over 15 years, in order to meet the requests from our clients, we have built cooperative relationships with our partner laboratories in South Korea, Taiwan, China, Singapore, Malaysia, Australia, the United States, Europe, and we have supported high-quality clinical trials conducted in each region.
Based on our highly evaluated technology and quality, we have received many requests for clinical trials in Japan from overseas pharmaceutical companies.
In global studies, when outsourcing work to overseas partner laboratories or dealing with overseas sponsors, mediford's experienced project managers (PMs) and study directors (SDs) communicate directly with them.
Project Managers/Study Directors with experience implementing a wide range of clinical trials targeting lifestyle-related diseases, oncology, central nervous system diseases, rare diseases, etc., will support you with wide technical information on clinical testing/measurement as well as extensive knowledge in every testing-related service such as sample collection materials, sample logistics and its storage.
We provide accurate proposals tailored to the content of each study, and proceed studies quickly and smoothly, from initiation stage through operation, to its completion.
To date, mediford has supported more than 50 studies, mainly in East Asia, collaborating with our overseas partner laboratories.
In outsourcing to overseas laboratories, mediford's quality assurance department, working with Project Managers/Study Directors, conducts on-site facility inspections, quality audits of testing and services, and regular assessments of our subcontractors to certify them and to assist high-quality services throughout long-term study periods.
Additionally, we investigate testing methodologies and service contents which are most applicable to each study in advance to ensure smooth preparation and operation.
The project manager/study director plays a central role in monitoring the progress of work at overseas laboratories, and additionally, the outsourcing management department regularly communicates with overseas laboratories through web meetings and written surveys to identify changes and problems. We will comprehensively understand such potential matters, and promptly respond to necessary matters, including sharing information with outsourcers.
Supporting areas for global studies at mediford
Experiences
-
Number of trials50+
-
Countries where clinical trials supported20+
-
Years of experience15+
-
Subcontracting laboratories15+
We want to plan a MRCT! However, …
Want to conduct high quality MRCTs.
This is the first MRCT for our company or We have little experience.
Have no candidate laboratories for outsourcing overseas…
Planning a long-period clinical trial. Worrying about management of study progress…
Worrying about communication with overseas laboratories…

Bothered about time differences among parties involved…
Worrying about management of central laboratory services alone…
Planning specialized testing, but worrying about the quality of newly established testing items...
Want to check the quality of MRCT, however, lacking professional knowledge…
Hesitaant about MRCT itself…
Mediford Corporation provides solutions for these various concerns!
Case study
At Mediford Corporation, we manage overseas laboratories on clients’ behalf. From initiation stage to operation stage, mediford’s staff will communicate with our partner laboratories to conduct the clinical trials. Here, we will introduce some of the examples we have carried out in cooperation with overseas laboratories.
Case 1 (Anticancer drug, Phase 3)
Project overview
This clinical trial was conducted in collaboration with one of China's leading laboratories (Central Laboratory), with mediford serving as the ordering party.
Subjects were gathered from more than 20 medical institutions in China, including Beijing and Shanghai. This clinical trial lasted for over five years, and for this whole period, mediford took all responsibility for progress monitoring of the project and communication with the laboratory in China.

Validation for development of test items
The protocol for this clinical trial included over 20 test items such as immunological tests and cytokines that were not on the testing menu of the laboratory in China, so development of new measurement systems (launching new test items) was necessary.
For this purpose, our lab team staff assisted the China laboratory, by providing the necessary support for the new launch, including reviews of validation test plans and reports, progress management, and other technical support.
Our expert staff constantly checked the status of subject registration (recruitment) and made suggestions regarding the best timing to conduct validation testing during the entire study period.
Our staff also visited the site to confirm the test-related data (so-called "raw data"). In regards to highly specialized fields, our technical department staff continuously supported our partner laboratory, by testing methodology proposals and technical training.
In addition, we perform all management* of the central laboratory services provided at our partner laboratory in China.
*Kit assembly of materials such as blood collection tubes and aliquot tubes, delivery of sample collection kits to medical institutions, performing of tests, reporting of test results, document storage necessary for new drug approval applications to the authorities, etc.
Quality management
Quality management of the services provided by the laboratory in China is carried out by mediford's department that specializes in overseas laboratories management.
Through monitoring by regular on-site visits and web meetings, we manage progress, constantly update information on tests and laboratory operating procedures, and check the implementation status of outsourced clinical trials and maintain its service quality.
Through this periodic monitoring, mediford aims to maintain and improve the quality of services by our outsourcing laboratories.
Case 2 (Rare disease, Phase 3)
Project overview
This trial, which targets a rare disease, required a wide range of cases/subjects from several South American countries.
We assembled material kits, including blood collection tubes for collecting sample, in accordance with the test conditions, and delivered the material kits to medical institutions in each country conducting clinical trials.
Sample collected in each country in South America were to be gathered in one country, where they would be stored for a specified period of time and then transported to Japan.

Communication with South America
The project management for this study was carried out in collaboration between mediford and our partner laboratory in South America.
Our Project Manager in Tokyo communicated day and night with the Project Manager located in Argentina, on the other side of the world, to proceed with the project. Regarding the material kit, we listened to the sponsor's requests in detail, investigated if materials such as blood collection tubes that met the requirements were available in South America, through the Project Manager in Argentina. Then we designed and produced material kits that met the sponsor's requests.
Then, utilizing the network of our partner South American laboratory, we delivered the completed kits to medical institutions in South American countries and arranged collected sample pickup and transportation to one country.
Quality management
Quality management of the services provided by our partner laboratories is carried out by mediford's department that specializes in overseas laboratories management. In addition to daily e-mail exchanges, we hold regular web meetings to manage progress, constantly update information on tests and project operation procedures, and keep up to date with the implementation status of outsourced projects.
Through regular communication, mediford aims to maintain and improve the quality of services by our outsourcing laboratories.